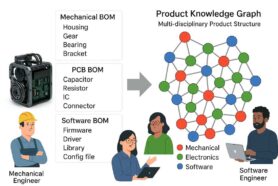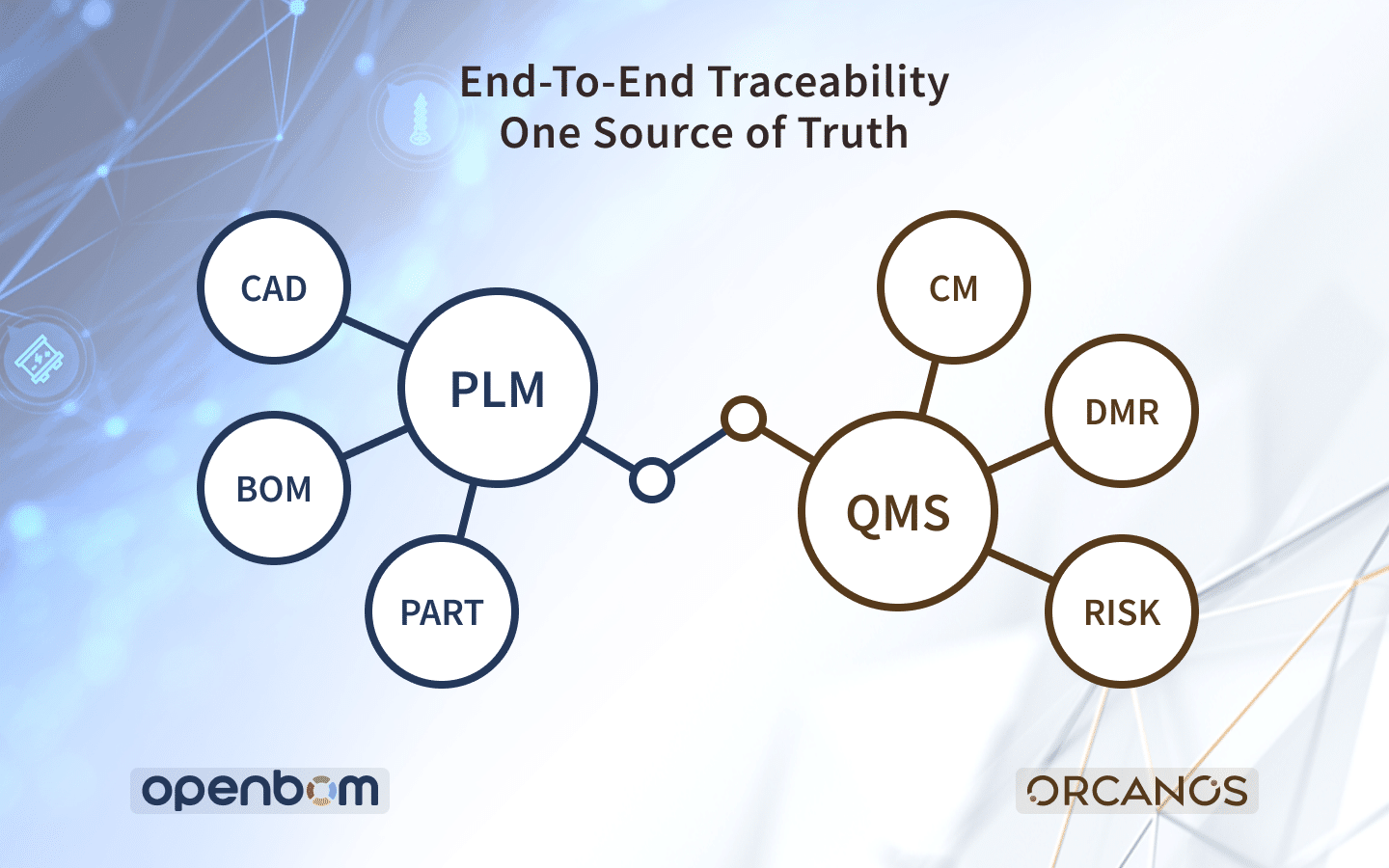
Quality Management Solutions (QMS) and Product Lifecycle Management (PLM) often go together. Still, the responsibilities of these two solutions usually belong to two siloed organizations. These applications can work independently, which typically means disconnected business processes and poor communication that affect the time-to-market outcome.
The FDA’s quality system requirements for all medical device companies are documented in the federal code of regulations under 21 CFR Part 820. Failing to comply with QMS standards can easily put your business at risk. Engineering processes can easily fail to incorporate process triggers from QMS and, on the other hand, quality processes may need more visibility on product data managed by the PLM system The solution is to connect PLM and QMS together to benefit from the visibility of information, streamlined processes, and increased efficiency.
I’m super excited to share the news about integrating two SaaS products – OpenBOM PLM and Orcanos QMS.
What is Orcanos QMS
As healthcare continues to evolve, medical device companies need to ensure that their engineering process and quality management processes can keep up with the fast pace of digital innovation. Business optimization strategies must now align with available technology, replacing archaic paper-based systems and spreadsheets with tools that provide a single view of the relevant information.
A key to compliance in the digital age is the removal of paper-based quality information and the integration of automated and optimized solutions.
Orcanos Quality Management System (QMS) is that tool. Our QMS software holds all of the required data points, for R&D compliance, needed to control all of the quality processes in a single access point. This focused view enables companies to eliminate the mistakes of paper trail in the quality journey and also allows insights to adhere to strict FDA regulations and established ISO/GMP quality standards.
Required reporting elements such as e-DMS, e-DHR, FMEA Risk Management, ECO, and Engineering which include Requirements Management, Verification & Validation, and Defect Tracking, are monitored in a single system.
Consolidating QMS channels means that companies can quickly ensure that products meet regulations and quality standards.
The Orcanos e-QMS platform is both easy to use and includes professional tools that build a centralized QMS within hours, such as:
- Electronic forms with e-Sign capabilities
- Tractability tool
- Support for ISO 13485, 21 CFR 820, ASPICE, and ISO 9001
- Automatic workflows for QMS forms
- A professional dashboard to track and control information
- Compliance virtual auditor engine that monitors data in real-time
How can an OpenBOM & Orcanos integration help your business?
Integrations with 3rd parties
PLM system organizes processes integrated with many other systems upstream and downstream. Those systems include Requirements engineering, CAD, ERP, MES, and Supply chain management.
BOM-QMS Traceability
A key foundation of any PLM system is Item and BOM management. It provides granular access to all product information, all revisions, and documents.
Integration with QMS adds a critical dimension to PLM solution – a direct connection between product and Quality related processes. Such integration allows the connection of the Bill of Materials with all related data to Quality data. It supports end-to-end traceability to the Engineering Change Order (ECO) downstream to the Device Master Record (DMR) used by the manufacturing plant and many other satellite processes.
One source of truth
PLM system provides a framework to manage product information and its lifecycle from the initial definition stage downstream through design, manufacturing, sales, and maintenance.
PLM system provides a single source of truth with all data needed to build a product, including CAD data, design information, specs, bill of materials, and all related information.
OpenBOM and Orcanos Integration Architecture
Both Oracnos and OpenBOM are modern SaaS platforms allowing seamless integration using REST API, automated event-driven mechanisms, and integrated visualization services. OpenBOM seamless integration with engineering and CAD resources allows all design information to connect with quality data while maintaining BOM as a central repository connected to quality data records management by Orcanos.
Conclusion:
OpenBOM and Orcanos integration provides comprehensive data management capabilities by combining product data records (eg. Bill of Materials) with Quality processes. In the PLM and QMS puzzle, such integration is crucial and complementary, allowing tracing records and processes.
The ability of OpenBOM to manage engineering information sources and Quality forms should inspire all our customers to think about a combined value.
Are you interested in integrated PLM and QMS systems? Register for free using the OpenBOM website and contact us to discuss more.
Are you interested in integrated PLM and QMS systems? Register for free using the OpenBOM website and contact us to discuss more.
Best, Oleg
Join our newsletter to receive a weekly portion of news, articles, and tips about OpenBOM and our community.